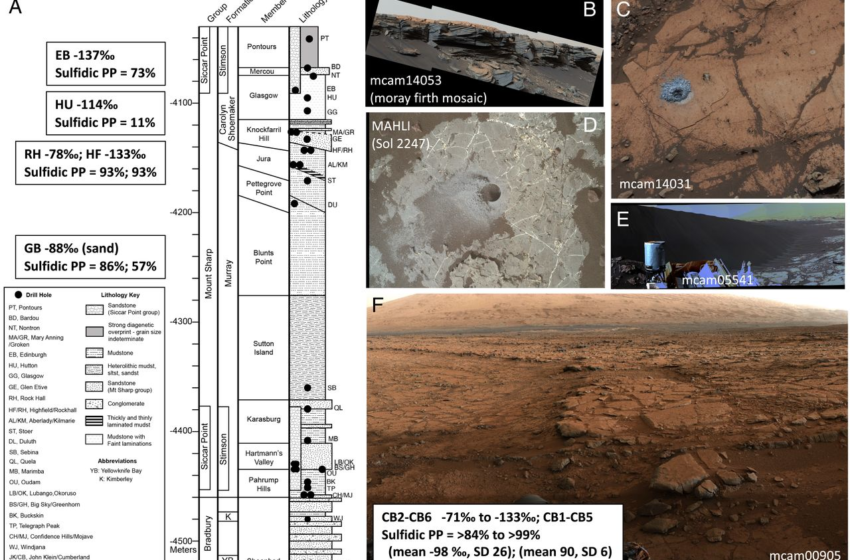
Carbon isotopic data from Martian sedimentary organic carbon can potentially elucidate the origin of indigenous organics and reveal aspects of the Martian carbon cycle. Extended exploration by the Mars Science Laboratory (MSL) Curiosity rover of the fluvio-lacustrine sedimentary system at Gale crater provides unique opportunities, as samples are collected from a variety of locations within a known stratigraphic context (1, 2). MSL has collected and analyzed more than 30 drilled samples on Mars between August 2012 and July 2021. These samples have been collected from varied lithologies from hundreds of meters of stratigraphy in Gale crater that represent the complex history and evolution of the region. A description of the mission samples studied can be found in the supplement, and a stratigraphic column is shown in Fig. 1A. Methods used in this work are also described in the supplement to this manuscript. This study considered the carbon isotopic values of methane evolved during pyrolysis as observed by the MSL tunable laser spectrometer (TLS) of the Sample Analysis at Mars (SAM) instrument suite (3) from 24 samples from Gale crater, Mars. Methane abundances and δ13C values were determined from analyzing TLS-SAM high-resolution spectra.
Discussion
Some of the 13C depletions reported here are anomalously large, especially with respect to the carbon isotopic composition of the Martian atmosphere, whose δ13C value reported earlier by TLS-SAM is ∼+46‰ (8). This atmospheric value reflects the integrated largescale loss of volatiles from the Martian atmosphere and thus, the δ13C composition of the atmosphere may have been less 13C enriched when Gale crater sediments were deposited. Because of the magnitude of the 13C depletions observed in CH4 evolved during EGA runs, the authors have considered potential rover-induced origins for the observations without uncovering any explanation. In fact, the TLS spectra obtained on Mars from evolved CH4 (SI Appendix, Fig. S1) are exceptionally clean and provide multiple 12C and 13C lines with which to calculate δ13C values, making it unlikely that the 13C depletions observed are due to an interfering organic molecule. From the repeat CB analyses, it appears that the isotope depletion observed in CH4 is most pronounced at lower temperatures. This observation suggests that precursors to the evolved CH4 are relatively volatile organic molecules. However, strong depletions were still observed in several samples using high-temperature cuts (>450 °C) in which the temperature cut includes a tail of a CH4 release centered at a lower temperature (e.g., SI Appendix, Fig. S2). The CH4 isotopic variation in CB samples, though, are not completely explained by differences in the temperature cut. Additionally, at Yellowknife Bay, the TLS analyses of CB using the 2.78-μm laser produced highly 13C-depleted CO2 δ13C values (SI Appendix, Table S1). While these TLS analyses of CB produced CO2 δ13C values that were, in some cases, comparable to CH4 δ13C (SI Appendix, Table S1), such depleted CO2 δ13C values have not been observed in later samples of the mission. In contrast, TLS CH4 δ13C values using the 3.27 μm laser show strong depletions in multiple different drill samples from vastly different parts of the mission. Because the observation of anomalous 13C values in evolved CH4 are repeatable with different samples spread out in space and time, we have focused on those values for this study.
We have considered the SAM instrument background of N-tert-butyldimethylsilyl-N-methyl-trifluoroacetamide (MTBSTFA (δ13C = −35‰; refs. 9 and 10) as a reasonable source of evolved CH4 to consider. Early in the mission, this background was estimated to contribute up to 900 nmol of CO2 during pyrolysis (11). In addition to oxidizing to CO2, MTBSTFA is known to react with water resulting in 1,3-bis(1,1-dimethylethyl)-1,1,3,3-tetramethyldisiloxane [or bisilylated water (BSW)], which can be monitored by the SAM QMS. Based on the levels of BSW detected, the level of MTBSTFA background has been variable between runs depending on how long it has been since a wet chemistry experiment and, for a few cases in which it was relevant, how long a sample was stored before analysis. For 16 of the samples, the mean of the total BSW observed during the course of the EGA runs was 1.0 nmol with an SD of 1.5 nmol (Table 2). HU had over 22 times this average, and Duluth (DU) had over 30 times more than this average. In the case of DU, the extreme amount of BSW observed was also concurrent with an anomalous amount of total EGA methane (774 nmol) and a TLS CH4 isotopic value (δ13C = −45‰) similar to MTBSTFA (δ13C = −35‰), which demonstrated that with elevated levels of MTBSTFA in the SAM background, a portion can end up as evolved CH4. Further, an intramolecular isotopic analysis of methyl-trifluoroacetamide from the hydrolysis of MTBSTFA showed that the types of carbon (methyl-carbon and carbonyl C) most likely to contribute CH4 from the MTBSTFA background were the most 13C enriched (SI Appendix, Fig. S3), making it unlikely that the observed anomalies stemmed from a site-specific carbon depletion obscured in the bulk δ13C value for MTBSTFA.
Major endmember carbon reservoirs (SI Appendix, Table S4) presently on Mars are the atmospheric CO2 (δ13C = +46 ± 4‰; ref. 8) and the igneous carbon (δ13C = −20 ± 4‰; ref. 12). TLS δ13C values between −17 ± 2‰ and −57 ± 2‰ were found for 10 samples including DU. The isotopic composition of the CH4 evolved during pyrolysis of these samples may reflect the MTBSTFA SAM background, Martian igneous carbon, and/or meteoritic infall along with any isotopic fractionations the occur during oven reactions. Laboratory experiments using solid materials were conducted to explore the magnitude of carbon isotopic fractionation possible during pyrolysis under conditions similar to SAM (SI Appendix, Table S2). We found that cleavage and reduction of methyl groups to CH4, as would happen for most CH4 derived from MTBSTFA products, produced little 13C depletion (0.4 to 4.6‰; SI Appendix, Fig. S4). CH4 evolved from recalcitrant sources (graphite and diamond) showed 13C-depletion of 8 to 21‰, CH4 from oxalate/oxamide showed moderate 13C depletion (25.0 to 25.3‰), and bicarbonate reduction showed the largest 13C depletions (28.5 to 49‰). Both our laboratory pyrolysis experiments (SI Appendix, Table S2) and modeling of possible isotopic pseudoequilibration during pyrolysis (SI Appendix, Figs. S5–S8 and Table S3) considering several different scenarios suggested that oven processes would typically produce fractionations less than 50‰ and, therefore, cannot account for the large 13C depletions observed in multiple samples at Gale crater. Applying the most extreme oven fractionation imagined to these carbon reservoirs results in CH4 with δ13C values of about −5 to −70‰. CB (CB1, CB2, CB3, CB5, CB6), Gobabeb (GB2), Highfield (HF), Rock Hall (RH), Hutton (HU), and EB showed TLS CH4 values more 13C depleted than this range, indicating that oven reactions are not likely to be causing their anomalous 13C-depleted values observed in evolved CH4.
It may be notable that the highly depleted 13C values for evolved CH4 have so far been found in five distinct locations at Gale crater, Mars (Table 2 and Fig. 1 A–F). The highly 13C-depleted signal was first seen in the mudstones of Yellowknife Bay on the crater floor (Fig. 1F) in a location where high thermal inertia values were measured from orbit and have been potentially attributed to secondary alteration at the end of the Peace Vallis fan (13). Next, highly 13C-depleted methane values were observed in a sample of the sand from the Bagnold dunes (GB2), a modern dune field of basaltic sand (Fig. 1E). Next, the depleted values were observed at the top of the Vera Rubin ridge (VRR) in mudstone samples of the red and gray Jura member of the Murray formation (RH and HF, respectively). Finally, the highly 13C-depleted values were observed just below the Basal Siccar Point group unconformity (HU; Fig. 1C) and in the overlying Stimson formation sandstone that forms the cap rock of the current Greenheugh pediment morphological feature (EB; Fig. 1B). These different locations include a variety of lithologies (mudstone, sand, and sandstone) and are temporally spread throughout the mission operations to date.
One potential connection between these evolved methane samples depleted in 13C is that they might all represent samples associated with a paleosurface (Figs. 3 and 4). The locations of the samples yielding depleted 13C methane along with their elevations are shown in Fig. 3 for illustration of this paleosurface possibility. Yellowknife Bay may have been weathered by flow out of Peace Vallis. The Bagnold dunes represent sand from, in part, a recently eroded local sandstone. The VRR is a resistant topographic ridge relatively higher than other samples of the Murray formation mudstone with a unique diagenetic history (14) that may include weathering from the outflow of the Gediz Vallis (6). Finally, the Greenheugh pediment is presently a large geomorphic surface and represents the remains of a larger paleosurface that would have extended downslope toward the crater floor (15). The HU sample, which shows the strongly 13C-depleted signature, is just below the Greenheugh pediment and is believed to have been exposed to fluid migration near the Basal Siccar Point group unconformity (7). Similar to the HU sample, Pontours (PT) is in the Carolyn Shoemaker formation below the Greenheugh pediment and, therefore, has likely been exposed to fluids during erosion of the pediment. PT is actually at a higher elevation than the EB sample because of the lateral slope of the pediment, and, interestingly, the TLS CH4 δ13C value for PT is intermediate between what is expected for SAM’s MTBSTFA background carbon and the highly 13C-depleted values (i.e., <−70‰) found for 10 of the Gale crater samples.
(A) Map of the northwest portion of Gale crater with annotations showing Peace Vallis and the alluvial fan leading toward the high thermal inertia region (High TI) in Aeolis Palus. Gediz Vallis is labeled to the south of the MSL traverse. The MSL traverse through sol 3192 is shown in red. The red rectangle outlines the region shown in B. Dashed line represents the profile in C. Base map is a mosaic from Calef and Parker (84). (B) Map of the MSL-specific study area and rover traverse through sol 3192. Samples analyzed by TLS with highly depleted 13C values are labeled along the traverse. Dashed line corresponds to the elevation profile shown in D. Base map is a HiRISE mosaic from the Planetary Data System (PDS) PLACES archive. (C) Profile from A to A′ in A showing the change in elevation from the lower end of Peace Vallis to Yellowknife Bay. (D) Elevation profile from B to B′ to B″ shown in B. Drill samples with highly depleted 13C values are labeled with approximate elevations.
Notional geologic history for Gale crater progressing from left to right starting with (A) a lacustrine environment. (B) After deposition, the lacustrine mudstones and sandstones were later exposed and eroded to produce the unconformity between the Mount Sharp group rocks (Murray and Carolyn Shoemaker formations) and the Stimson formation. (C and D) The Stimson sandstone has also been eroded to produce a paleosurface (and later the present landscape of ridges, buttes, and a pediment).
While this distribution of samples yielding a strongly 13C-depleted signature may be a coincidence, it could be explained if the signature relates to a process that primarily impacted a paleosurface associated with outflow from Gediz Vallis and Peace Vallis that included the Greenheugh pediment capping unit, the topographic high represented by the VRR, and the crater floor at Yellowknife Bay. Given this distribution of samples yielding isotopically anomalous methane, we have considered multiple phenomena that have the potential to explain the results. The phenomena discussed are not presented in order of preference, but rather are treated equally, starting with the canonical explanation for when this type of geochemical signature is found on Earth and moving to possible processes that would be increasingly specific to Mars.
Martian Methanotrophy?
The observed 13C-depleted EGA CH4 was presumably released from organic materials in the solid sample during pyrolysis. The canonical explanation on Earth for highly depleted carbon associated with a paleosurface would be microbial methanotrophy converting methane into biomass when the methane was already 13C depleted because of its biological production during methanogenesis (e.g., ref. 16). The Archean Tumbiana Formation has highly 13C-depleted biomass preserved along with abundant lacustrine stromatolites (e.g., refs. 17 and 18). The bulk biomass found in the Tumbiana Formation has δ13C values as low as −60‰ (19), the origin of which is often attributed to widespread methanotrophy during a period of abundant methane in the Earth’s atmosphere and shallow basins (16, 20). Highly depleted biomass is also found in some sediments in and around active modern marine methane seeps (e.g., refs. 21⇓⇓–24), and there are several examples of highly 13C-depleted biomass preserved in paleo seeps deposits (25, 26).
Because of both the overall oxidizing state of the Martian atmosphere and the presence of ferric iron and sulfate minerals, during times of large methane releases, methanotrophy would be energetically favorable. Based on observations of apparent methane plumes from the Martian interior and the growing understanding of the ways methane can be microbially oxidized on Earth, microbial methanotrophy has been proposed as a possible metabolism for recent and ancient Mars (27, 28). This explanation would also potentially explain the observation of reduced sulfur in many of the same samples in which negative δ13C values were found (29) because marine methane oxidation is coupled to sulfate reduction. If the highly 13C-depleted carbon and reduced sulfur are indeed found predominantly together on a paleosurface, the co-occurrence could result from the coproduction of 13C-depleted microbial biomass and sulfides during the microbial anaerobic oxidation of methane based on sulfate as an electron acceptor.
In order to get the observed magnitude of 13C depletion in the observed EGA methane released from organic materials, the CH4 that was originally consumed by microorganisms in this model would need to already be highly 13C depleted (perhaps −40 to −100‰) because the observed depletion on Earth during anaerobic methanotrophy is on the order of about 30‰ (30), assuming fractionations associated with possible Martian metabolisms are similar to those observed on Earth. Therefore, this explanation requires multiple steps with the CH4 consumed by the methanotrophs to be biological CH4 from microbial methanogenesis. Furthermore, the inorganic CO2 fueling this microbial ecosystem would need to have a δ13C composition similar to Martian magmatic carbon (−20‰; refs. 12 and 31) rather than the highly 13C-enriched values observed in the present Martian atmosphere (+46‰; ref. 8).
For this model to work, either the deposition of the 13C-depleted carbon would have needed to occur before the loss of significant carbon from the Martian atmosphere or there would need to have been CO2 reservoirs in the Martian subsurface that were isolated from the atmosphere. In either scenario, the initial carbon could have had an isotopic composition down to −20‰. The first option, however, appears inconsistent with Gale crater sedimentology, given the relatively late emplacement of the Stimson formation that is cut by the paleosurface of interest here. The other option is possible, but it is hard to evaluate with our limited knowledge of the hydrodynamics of subsurface Mars. Potentially supporting this model is the observation of long, straight alkanes in the CB sample (32). Overall, there is, however, no supporting sedimentological evidence for microbial methanotrophy on the paleosurface discussed here. This is in sharp contrast to analog methanotrophic environments on Earth, for which there is often plenty of textural evidence for microbial processes in deposited layers. It is plausible that such evidence once existed at Gale crater as an authigenic carbonate (e.g., refs. 33 and 34) that was later dissolved by acidic fluids. While sedimentary structures may be destroyed by dissolution, dissolution can concentrate molecular biosignatures. The Curiosity rover will again encounter the Stimson formation sandstone on the Greenheugh pediment, providing an opportunity to search for specific organic molecules (e.g., refs. 35 and 36) in this rock unit. The lack of sedimentary evidence for microbial surface activity and the need to avoid influence from a 13C-enriched Martian atmosphere cast enough doubt on this biological explanation of surface methanotrophy to tentatively dismiss it pending further exploration for either evidence of surface-associated microbial activity or microbial-influenced sediments whose organic material could have been redeposited to the paleosurface.
Interstellar Dust?
The solar system passes through an average-sized giant molecular cloud (GMC) once every 100 million years, providing a mechanism for triggering cooling events on terrestrial planets through the influx of particles to planetary atmospheres that is substantially higher (20 to 100 times) than the ongoing flux of interplanetary dust (37). The solar system passing through a dense molecular cloud would inevitably also result in an influx of 13C-depleted carbonaceous particles (38), as about 1% of such interstellar clouds is dust (39). The δ13C value for interstellar dust in the Allende meteorite has been shown to be as low as −260‰ (40), demonstrating that interstellar dust is a potential source of highly depleted carbon for periodic deposition on the surface of Mars. It is also plausible, but presently uncertain, that these particles would similarly be associated with sulfides having negative δ34S values, as observed in the Curiosity data. The flux of such particles would be quite low and typically diluted by other sources of carbon. However, because the arrival of such particles is predicted to trigger a global cooling event, it is reasonable that particles would accumulate on the surface of glacial ice largely absent of typical sedimentary carbon. Glacial melt during the glacial period and ice retreat after should leave the interstellar dust particles on the glacial geomorphological surface. The existing sedimentological evidence for the paleosurface that has cut the Stimson formation does not rule out glacial processes, with Curiosity having only visited the Greenheugh pediment surface briefly at EB, and some studies across Mars support such an interpretation for similar outflow channels (41⇓⇓–44).
This explanation of interstellar dust as a source of the observed 13C-depleted values at Gale crater largely fits the observations if the isotopic signatures are not diluted by other geological processes and with the understanding that this explanation relies on a rare event, making it perhaps coincidental that the mission should find this signature preserved. Finally, the SAM results presented here suggest that the most 13C-depleted values are liberated during relatively low oven temperatures, with the high-temperature examples typically being tails of lower-temperature methane release. These observations might be understood to indicate that the organic precursor molecules being converted into methane in the SAM oven are not recalcitrant kerogen-like molecules, as pyrolysis of kerogen usually occurs at a higher temperature. If this interpretation is correct, this constraint can be consistent with an interstellar dust origin, as there are a wide variety of known interstellar molecules, including many that are highly volatile (45). Overall, this explanation is plausible, but it requires additional research outside the scope of this report to verify, including the δ34S values and the nature and relative amounts of carbon forms in GMC dust.
Abiotic Reduction of CO2?
There is growing recognition of the possible abiotic production of organic carbon on Mars through either electrochemical reduction (46) or photochemical reduction (10). In both cases, CO2 would be converted into organic material abiotically and therefore could, in principle, lead to 13C-depleted organic material associated with a paleosurface. For electrochemical reduction, sulfides are one of the various reduced minerals that thermodynamically drive such carbon fixation reactions, and, similarly, sulfides are possible mineral catalysts that can promote photochemical reduction of CO2. Thus, if abiotic reduction of CO2 is an important process on Mars, it is likely to be associated with reduced sulfur, as observed at Gale crater. The present atmosphere of Mars, as previously noted, is highly enriched in 13C (+46‰), and so either the abiotic reduction would need to have been occurring in subsurface environments (which does not match our observations) or would need to have occurred prior to the loss of much of the Martian atmosphere (which appears somewhat inconsistent with the timing of the erosional paleosurface with which the depleted carbon appears to be associated). While the timing of the erosional paleosurface is poorly constrained to the Hesperian, deuterium enrichment of clays at Gale crater indicated significant loss of atmospheric volatiles by this time (47), and the implications of that loss for the overall history of Martian volatile loss is an area of active research (48). Overall, though, the carbon isotopic fractionations expected during abiotic reduction are likely similar to those measured for abiotic hydrothermal reduction (up to ∼50‰; SI Appendix, Fig. S9B). Fractionation of this magnitude would not produce organic material as depleted as the observed TLS values for the EGA methane reported in Table 1, and therefore, this explanation does not appear to explain the observations of highly 13C-depleted evolved methane during pyrolysis of samples at Gale crater. However, carbon isotopic fractionation during photochemical reduction of CO2 on a mineral surface has not been experimentally evaluated, and so, further research is needed before we can fully evaluate the abiotic reduction as the source of the highly 13C-depleted carbon isotopic values reported here. To be clear, there are a number of other CH4 isotope values reported in Table 1 that could reflect the reduction of CO2 on the Martian surface, including the most 13C-enriched values (−1 ± 6‰ to +22 ± 10‰). These 13C-enriched results match what would be expected for processes, biotic or abiotic, that produce organic material from the modern Martian atmosphere, for example.
Photolysis of CH4 or CO and SO2?
Photochemical reactions are attractive as a source for the observed phenomenon because photochemical reactions are known to have influenced Mars’s volatiles, including the sulfur isotopic values found in Martian meteorites (49⇓⇓–52). Also, the fallout of products from atmospheric photochemistry might be expected to accumulate on an erosional surface, such as the proposed paleosurface discussed throughout this paper. There are several different overall schemes by which photochemistry in the Martian atmosphere might result in the large δ13C depletions observed by the MSL-TLS component of the SAM instrument.
A growing number of studies have reported variable CH4 abundances in the Martian atmosphere (53⇓–55), including recent reports of seasonal and diurnal variation (56, 57) and plumes (55) detectable on the surface at Gale crater. The photolysis of atmospheric CH4 in a relatively dry, anaerobic atmosphere can produce organic aerosols and particles (e.g., Ref. 58). Perhaps when the postulated paleosurface was exposed, CH4 photolysis resulted in the deposition of such organic material. This model would explain why the highly depleted δ13C values seem to be associated with a possible paleosurface, similar to a model proposed for the Late-Archean Earth (59). Further, the model could potentially also explain the observation of 34S-depleted reduced sulfur in the same deposits if there was also SO2 photolysis along with the CH4 photochemistry producing sulfur-containing organic molecules. For example, Halevy (60) suggested that photoexcitation of SO2 can lead to the production of methanesulfonic acid on the Archean Earth. Additional work is needed to explore the possibility that CH4 photolysis during Martian plume events would deposit 34S-depleted sulfur-containing organic material. However, past studies indicate that the magnitude of carbon isotopic fractionation during CH4 photolysis is relatively small (<15‰; refs. 61⇓–63). Fractionation during CH4 photolysis appears insufficient to produce δ13C values as depleted as some of those observed at Gale crater even when starting from bulk Mars δ13C values (−20‰) representative of carbon from the interior of Mars. In order to produce organic material that is highly 13C depleted (considerably more depleted than −70‰), another step with significant δ13C fractionation would need to have occurred during the formation of the methane. In principle, this could be abiotic CO2 reduction in the Martian crust during serpentinization or microbial methanogenesis. As noted previously, the abiotic reduction of CO2 results in ∼50‰ 13C depletion. If the source CO2 had a δ13C composition of −20‰, subsequent abiotic reduction to CH4, and subsequent photochemistry, then the resultant organic material deposited could be as depleted as perhaps −85‰, a value that does not adequately include all of the values observed at Gale crater. If microbial methanogenesis were producing the observed atmospheric CH4, photolysis of the biologically produced CH4 would result in isotopic compositions consistent with the strongly depleted 13C values observed. At a minimum, however, new observations would be needed to confirm a biological origin for Mars CH4 plumes before this explanation can be accepted.
Alternatively, photochemical reduction of CO2 to formaldehyde (CH2O) via CO as an intermediate (e.g., ref. 64) might be responsible for producing a 13C-depleted organic material because the photochemical partitioning of CO2 and CO can lead to 13C depletion in the CO relative to CO2 (65⇓–67). However, most past studies have considered Martian CO to be isotopically similar to CO2, with any 13C depletion relative to CO2 to be on the order of about 30‰ (68). Recently, however, photodissociation of CO has been shown to yield hundreds of per mil 13C enrichments in the resultant CO2 at 70 K using vacuum ultraviolet (VUV) radiation around 100 nm from a synchrotron source (69), while photodissociation of CO2 has been shown to yield tens of per mil 13C enrichments in the resultant CO (70, 71). While these experiments use different ultraviolet (UV) wavelengths and different temperatures, together they illustrate how photochemical partitioning in the Martian atmosphere might result in anomalously 13C-depleted CO that could influence geochemistry observed at the Martian surface (72). In fact, CO photolysis has been calculated to have large carbon isotopic fractionation (with a fractionation factor of 0.6) at the top of the Martian atmosphere (73). VUV radiation would interact with the upper Martian atmosphere, and the presence of CO in the Martian atmosphere is maintained by CO2 photolysis. If Martian photochemistry resulted in 13C-depleted CO throughout the atmosphere in the past, photoproduction of formaldehyde and other organics from CO could have accumulated on the exposed surface at Gale crater, resulting in the isotope results reported here (67, 74). Obstacles to the deposition of organic material from the photolysis of CO2 have been the low yield and photochemical instability of the formaldehyde produced (75). Photochemically produced formaldehyde on Mars might react quickly with SO2 to yield hydroxymethanesulfonate, protecting it from photolysis back to CO (76), and it is plausible that elevated levels of atmospheric SO2 would directly shield formaldehyde because of the spectral overlap of SO2 and CH2O photoabsorption cross sections combined with the greater strength of SO2 absorption (77, 78). After major eruptions, when there were higher levels of CO, SO2, and H2, the deposition of organics should be maximized, including perhaps formaldehyde, hydroxymethanesulfonate, carbonyl sulfide (79), and thioformaldehyde (80). Because of a relatively long photochemical equilibrium lifetime of SO2 of ∼1 Mars year (75, 81) and photodissociative shielding of CH2O afforded by SO2, the likelihood of such reactions increases. Incorporation of 13C-depleted organics into frost or glacial ice at the Martian surface could also concentrate these photochemical products and lead to their preservation in the local soil, protected from further UV reactions. However, the VUV wavelength radiation shown to produce large fractionation is too short for most of the Martian atmosphere and has yet to be explored at various temperatures, limiting any conclusions that can be drawn here. Also, direct observation of CO in the Martian atmosphere by spectroscopy from Earth has not revealed a large 13C depletion in CO, with the results appearing to be within 250‰ of the telluric value (82) and probably slightly 13C enriched relative to Earth (66, 68). Without further information with which to deconvolve the details of where and how carbon was fractionated, we can conclude the photochemical production of organic material from 13C-depleted CO is a possible scenario on Mars for deposition of highly depleted organic material on to an exposed surface (74) that should not be rejected without further investigation of Martian CO and the photochemical processes that influence its carbon isotopic composition.
Methods
Samples.
The first drilled MSL samples were collected from the Sheepbed member mudstones in Yellowknife Bay. The Yellowknife Bay region in Gale includes portions of high thermal inertia rocks toward the distal end of the alluvial fan from Peace Vallis. The first and second samples of Sheepbed mudstones—John Klein and CB, respectively—were taken to investigate rocks deposited in this fluvial-lacustrine context. Analyses of these samples indicated the presence of an ancient habitable environment on Mars (2).
After analysis of the Sheepbed mudstones, the rover continued its traverse through Aeolis Palus and then began its ascent of Mt. Sharp. Starting in September 2014, the rover transitioned from Bradbury group rocks to Mt. Sharp group rocks, which consist of hundreds of meters of sedimentary stratigraphy subdivided into the Murray formation and the Carolyn Shoemaker formation. The Murray formation comprises the basal unit of Mt. Sharp and is divided among several distinct lithologic members dominated by mudstones. The Carolyn Shoemaker formation conformably overlies the Murray formation and has prevalent sandstone lithology. To date, more than 20 drilled samples of Mt. Sharp group rocks have been collected and analyzed from the various members. Included among these samples are analyses of regions that show unique properties from orbit such as the VRR, the Glen Torridon region, and the lower sulfate-bearing unit. VRR is a prominent topographic high on lower Mount Sharp that had orbital evidence for elevated hematite and was determined to have formed from several diagenetic events (14). South of the ridge is the Glen Torridon region, which was notable for its elevated orbital spectral signature of phyllosilicates. The most recent samples (as of July 2021) have been documenting the transition from clays to hydrated sulfates.
In a few locations along the rover’s traverse, samples have been taken from the Siccar Point group, which is comprised of the Stimson formation sandstones. The Stimson formation unconformably overlies the Mt. Sharp group and represents the remnants of an ancient, lithified dune field. The first four samples of Stimson formation rocks were taken to study both the parent rock and areas of localized alteration. The fifth sample of Stimson formation was taken later in the mission, during the Glen Torridon campaign, when the rover ascended the Greenheugh pediment.
In addition to the drilled samples, MSL has analyzed scooped samples of sand. The fine sand-sized GB and Ogunquit Beach samples were taken from the active Bagnold Dune field and represent local modern aeolian processes.
MSL EGA and MSL Carbon Isotopic Analysis.
The SAM consists of a suite of instruments including two pyrolysis ovens, a QMS, six gas chromatography columns, and a TLS (3). Together, SAM can measure the volatile composition of solid samples on Mars through thermal decomposition experiments. EGA–mass spectrometry (EGA-MS) heats samples to ∼850 °C and sends evolved volatiles directly to the QMS for identification with a constant flow of He. In this paper, QMS was used for the quantification of sulfur gases, as well as BSW (see SI Appendix, Supplementary Text for additional details). As explained further in the supplement, quadratic discriminant analyses of the QMS gas results provided posterior probabilities for whether each drill sample contained reduced sulfur (6). Gases during EGA can be sampled by the TLS within a specified temperature range. The TLS uses several specific infrared wavelengths to quantify H2O, CO2, and CH4 as well as the isotopes of these gases when present in great enough abundance.
For SAM EGA runs, the solid sample is contained in an oven whose temperature is increased at 35 °C/min to ∼850 °C while helium (0.8 sccm, 25 mbar) is flowed through the pyrolysis oven. After evacuation of the TLS sample (Herriott) cell to record empty cell values, the TLS cell is opened to receive a “temperature cut” ingest (e.g., 200 to 350 °C) during EGA that introduces He and evolved volatiles into the cell, which is then sealed off for analysis. Typically, empty-cell methane abundances are extremely low (parts per billion by volume) compared to the full-cell EGA values (tens of parts per million by volume [ppmv]) so that empty-cell corrections are not needed. Full-cell pressures (mainly helium) are typically 5 to 15 mbar.
As further discussed in the supplement, three strong 12CH4 lines and four strong 13CH4 CH4 lines are identified in the recorded TLS spectra, although only two of the 13CH4 lines are chosen for analysis to minimize water interferences (SI Appendix, Fig. S1). With the cell closed, the laser scans over the methane lines every second, and on-board, TLS captures average spectra over sequential 2.7-min periods that are downloaded. Analysis of each of these 2.7-min spectra is done on a line-by-line basis through comparison with HITRAN 2016 calculations (that employ terrestrial δ13C values, so that volume mixing ratios in ppmv are retrieved for each of the three 12CH4 and two 13CH4 lines. In some cases of very high mixing ratios, the strongest 12CH4 is very deep and not used. Then, the average values of the retrieved volume mixing ratios for 12CH4 and for 13CH4 are calculated from each of the 2.7-min spectra. During the run, 26 full-cell spectra are recorded, so that the 26 values retrieved of average abundance values can be statistically analyzed to find the mean value with SEs of either 67% CI or 95% CI. Ratioing the 13CH4 mean abundance with that determined for 12CH4 provides the δ13C values reported here normalized to the terrestrial PDB reference expectation such thatδ13C=((C13C12) Sample(C13C12) PBD std.−1) × 1,000 ‰,where PDB std. is Pee Dee Belemnite standard, which is equal to 0.0112372.
Supporting Laboratory Analyses.
As discussed in the SI Appendix, Supplementary Text, laboratory analyses were conducted (SI Appendix, Figs. S3 and S4, and Table S2) in support of this paper, including an NMR-based investigation (83) of the intramolecular isotopes of methyl-trifluoroacetamide, part of the MTBSTFA molecule. Laboratory studies were also used to study carbon isotopic fractionation during pyrolysis to CH4 under conditions similar to that of the SAM instrument. Various carbon-containing materials were placed in silver boats and dropped into an oven at 400 °C under flowing helium. The oven temperature then increased to >850 °C, and evolved gases, including CH4, were trapped out of the He flow between 455 °C and 755 °C to simulate a high-temperature TLS cut.